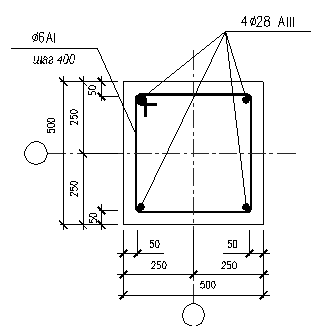
Armirovanie Monolitnoj Kolonni Chertezh
Transport Introduction We work to promote sustainable transport which is safe, clean and competitive, through the development of freight and personal mobility by inland transport modes, by improving traffic safety, environmental performance, energy efficiency, inland transport security and efficient service provision in the transport sector. Citation: Fisher MA, McKinley KL, Bradley LH, Viola SR, Hecht MH (2011) De Novo Designed Proteins from a Library of Artificial Sequences Function in Escherichia.
Abstract A central challenge of synthetic biology is to enable the growth of living systems using parts that are not derived from nature, but designed and synthesized in the laboratory. As an initial step toward achieving this goal, we probed the ability of a collection of >10 6 de novo designed proteins to provide biological functions necessary to sustain cell growth. Our collection of proteins was drawn from a combinatorial library of 102-residue sequences, designed by binary patterning of polar and nonpolar residues to fold into stable 4-helix bundles. We probed the capacity of proteins from this library to function in vivo by testing their abilities to rescue 27 different knockout strains of Escherichia coli, each deleted for a conditionally essential gene. Four different strains – Δ serB, Δ gltA, Δ ilvA, and Δ fes – were rescued by specific sequences from our library. Further experiments demonstrated that a strain simultaneously deleted for all four genes was rescued by co-expression of four novel sequences.
Thus, cells deleted for ∼0.1% of the E. Coli genome (and ∼1% of the genes required for growth under nutrient-poor conditions) can be sustained by sequences designed de novo. Citation: Fisher MA, McKinley KL, Bradley LH, Viola SR, Hecht MH (2011) De Novo Designed Proteins from a Library of Artificial Sequences Function in Escherichia Coli and Enable Cell Growth.
PLoS ONE 6(1): e15364. Editor: Mark Isalan, Center for Genomic Regulation, Spain Received: August 26, 2010; Accepted: November 11, 2010; Published: January 4, 2011 Copyright: © 2011 Fisher et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was funded by NSF grant MCB-0817651 (URL: ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing interests: The authors have declared that no competing interests exist.
Introduction In 1906 Jacques Loeb suggested that the synthesis of life is a significant goal of biology. A century later, the construction of natural genomes from off-the-shelf chemicals, demonstrates significant progress toward achieving this goal. Until now, however, most advances in synthetic biology have relied on collections of parts – genes, proteins, and regulatory elements – derived from sequences that already exist in nature.  Must the toolkit of life be so restricted? Natural sequences comprise only a miniscule fraction of the theoretical sequence space that is possible for genes and proteins. Indeed, a collection containing just a single molecule of every one of the 20 100 possible 100-residue proteins would fill a volume larger than a mole of universes.
Must the toolkit of life be so restricted? Natural sequences comprise only a miniscule fraction of the theoretical sequence space that is possible for genes and proteins. Indeed, a collection containing just a single molecule of every one of the 20 100 possible 100-residue proteins would fill a volume larger than a mole of universes.
From this enormous potential for diversity, natural selection has operated over billions of years to yield a relatively small collection of sequences: Life is sustained by only ∼4,000 genes in E. Coli and approximately 5-fold more in humans,. These considerations might lead to the supposition that genes and proteins capable of sustaining life are somehow “special.” Is this true? Or might we find functional molecular parts in a collection of artificial sequences designed “from scratch” in the laboratory? To address these questions, we probed the ability of unevolved sequence space to encode functions that enable cell growth. We designed and constructed a collection of artificial genes encoding approximately 1.5×10 6 novel amino acid sequences.